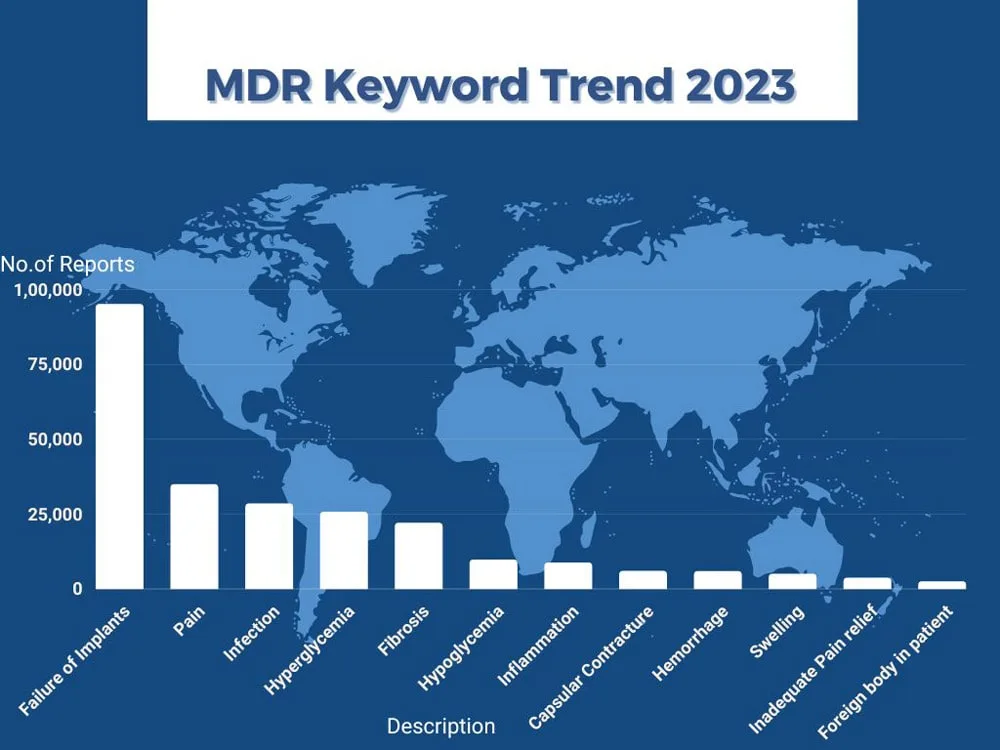
Smarteeva Unveils MDREngine: A Cutting-Edge Medical Device Analytics Platform for All Skill Levels

Smarteeva releases its Winter 2020 Smart Complaint System to provide full EUMDR compliance for Medical Device Companies

Smarteeva continues to innovate, investing heavily in the integration of their customers’ Customer Service and Complaint Handling processes
MDR with Cerebrovascular Accidents/Strokes have been increasing especially in the past 3 years
Smarteeva Platform Data Sheet
The Smarteeva Platform is the engine powering all Smarteeva Products. A modern intelligent platform built...
Smart Audit Trail Data Sheet
Smarteeva’s Smart Audit Trail organizations can quickly research and asses the state of their regulatory...
Field Notification Management
Smarteeva Field Notification Management provides end to end Notification Management functionality. Our next-gen solution delivers...
Risk Management Data Sheet
Smarteeva’s Risk Management Solution embeds a state of the art approach to managing risk in...
Customer Portal Data Sheet
Rapid globalization coupled with Todays intelligent customer require tighter collaboration across your organization through to...
Complaints Management Data Sheet
Complaint Solution provides the next generation of complaints and investigation management by fully integrating risk...
Smarteeva announces the release of the Smarteeva Platform for the Medical Device Industry
Santa Barbara, CA – Jan 10, 2019 – Smarteeva, Inc announced the recent release of the...
Smarteeva announces the release of its Summer 2023 Post Market Surveillance Suite
Smarteeva, a premier post-market surveillance partner to global medical device companies, is excited to announce...
Top 5 Trends in Post-Market Surveillance 2023
At Smarteeva, we interact with numerous medical device industry insiders and pay attention to where...
Patient Deaths decreasing as percentage of MDRs in 2022
The FDA requires Medical Device Reports (MDRs) submission within 30 days of Complaint Awareness and...
Top MDR submitters in 2022
Smarteeva maintains an internal database of medical device adverse events. We use public data such...
The FDA received over 3 million MDRs in 2022
The Increase in the Volume of Complaints and MDR reports In the past 10 years,...
EU Incident Reporting by Smarteeva
Smarteeva delivers state-of-the-art simplicity with its newly released Manufacturer Incident Report for EU MDR
Smarteeva Post Market Surveillance and EU MDR Ready Suite
Smarteeva’s Postmarket Device Lifecycle suite of products provides an unsurpassed amount of efficiency with the...
Smarteeva Audit Trail – Quick Demo
Smarteeva’s Smart Audit Trail takes record tracking to the next level of usability and productivity....
Smarteeva – Smart Minute – Decision Trees
Smarteeva delivers smart decision trees for reportability assessments, questionnaires, guided forms, and checklists.
Smarteeva – Smart Minute – Smart Audit Trail
Smarteeva Changes How Medical Device Companies utilize Audit trails.