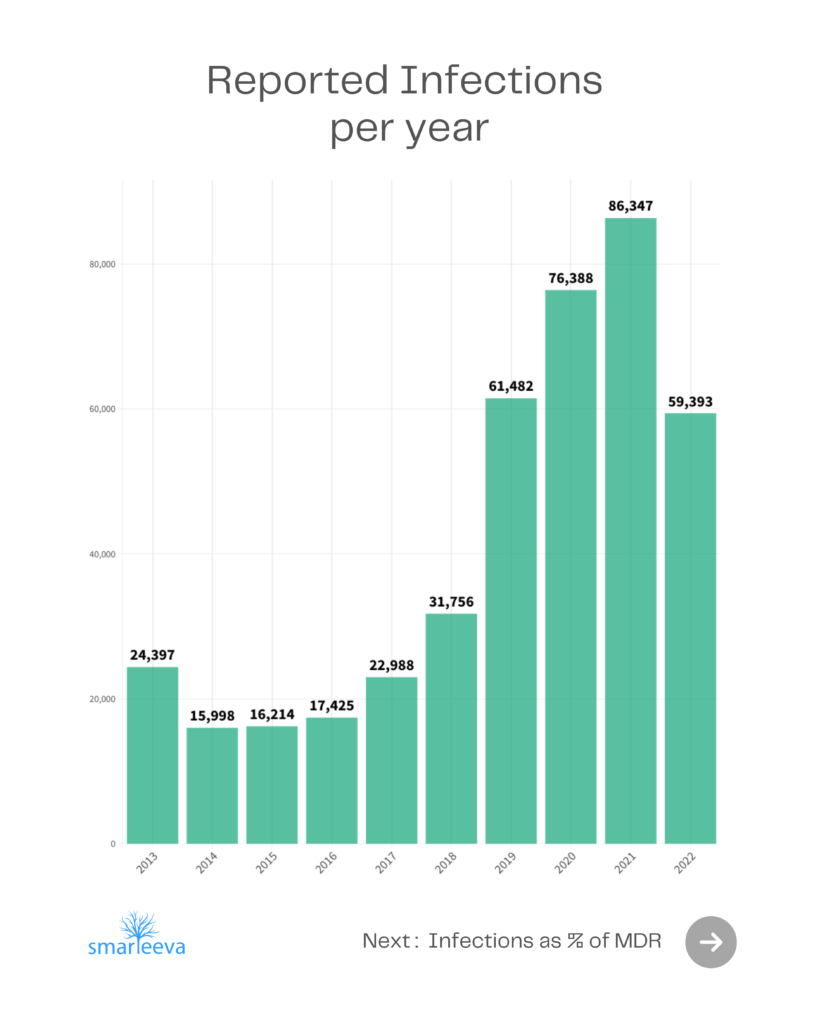
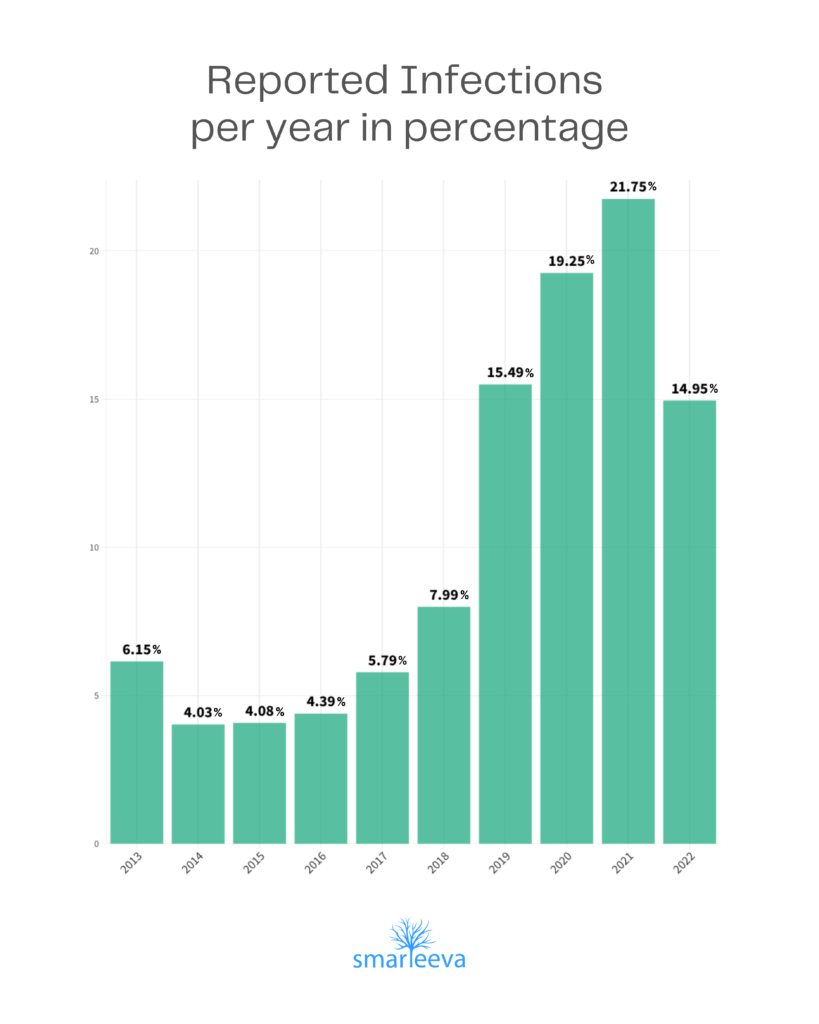
Medical Device related Patient Infections until 2022
When manufacturers prepare Medical Device Report (MDR) submissions to the FDA, they collect data from healthcare providers. The information from health care providers sometimes mentions that a patient had an infection during the course manufacturer’s medical device usage. The cause of the patient’s infection might or might not have anything to do with the device. Nevertheless, the manufacturer is required to submit that information to the FDA.
See how Smarteeva can help you make Post Market Surveillance faster and easier by going to our Product Page
About Smarteeva:
Based out of Santa Barbara, CA. Smarteeva Software provides cutting-edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user-friendliness. Built on the Salesforce.com Platform and utilizing the latest AI and Machine Learning innovations, Smarteeva Software is a true partner to its business users.