
We are trying to find major keyword patterns across device problems and patient problems that are also present in the B5 and H10 text of MDR reports
The most frequent keywords are tracked using counters
This exercise is also done by year to see any consistencies or major shifts in trends of the problems reported
Some boilerplate terms such as patient, device, clinical, use, available, adverse and event are not considered in this exercise
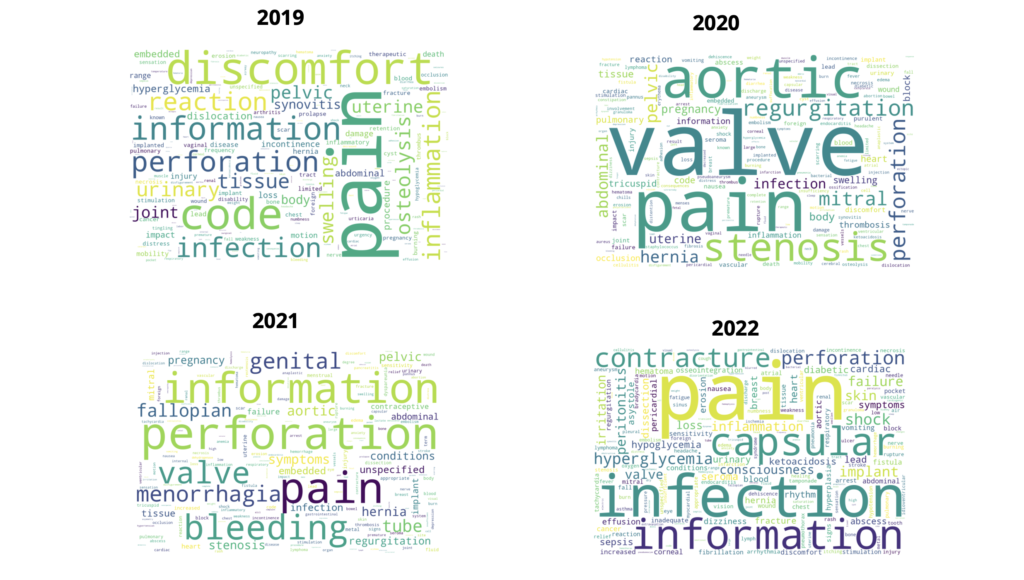
See the full Report Analysis here: Full Patient Problem Report Analysis
About Smarteeva:
Based out of Santa Barbara, CA. Smarteeva Software provides cutting edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user friendliness. Built on the Salesforce.com Platform and utilizing the latest innovations in AI and Machine Learning, Smarteeva Software provides a true partner to it’s business users.

