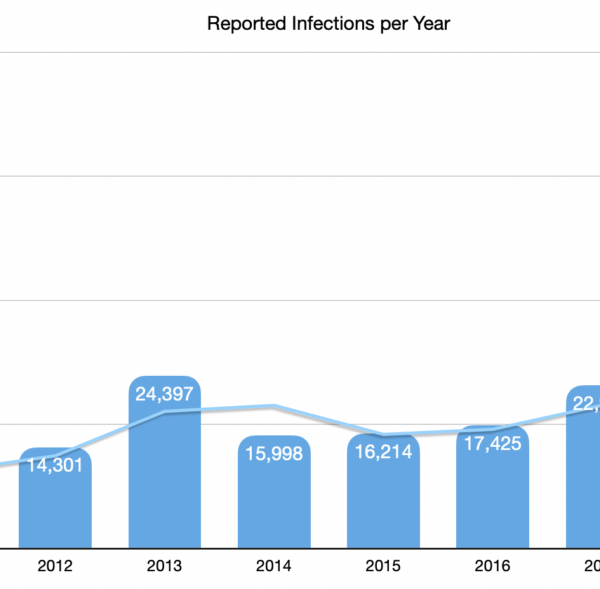
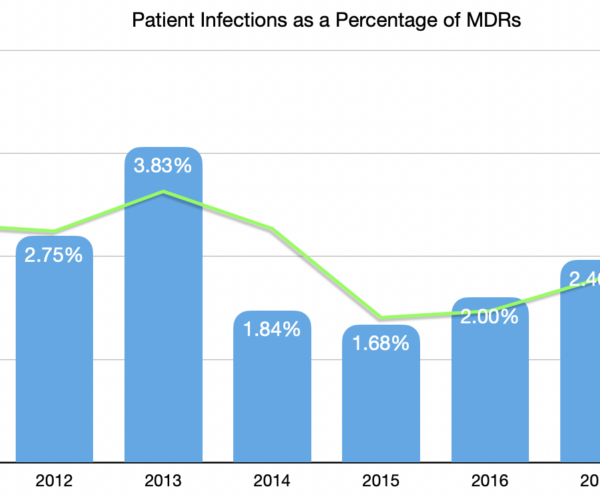
Patient Infections reported to the FDA are showing an increase in both absolute and relative numbers. Specifically, over the past 10 years the number of infections reported has increased an average of 28% annually. If you narrow the range to the past 3 years only, the rate of increase jumps to an average of 55%.
When manufacturers prepare Medical Device Report (MDR) submissions to the FDA, they collect data from health care providers. The information coming from health care providers sometimes mentions that patient had an infection in the course of using the manufacturer’s medical device. The cause of the patient infection might or might not have anything to do with the device, nevertheless the manufacturer is required to submit that information to the FDA.
The numbers reported to the FDA in the past 10 years have shown a consistent increase with the exception of the 2015/2016 calendar years.
See how Smarteeva can help you make Post Market Surveillance faster and easier by going to our Product Page
About Smarteeva
Based out of Santa Barbara, CA. Smarteeva Software provides cutting edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user friendliness. Built on the Salesforce.com Platform and utilizing the latest innovations in AI and Machine Learning, Smarteeva Software provides a true partner to it’s business users.

