Medical Device Reports (MDRs) are typically required to be submitted to the FDA within 30 days of Complaint Awareness. FDA utilizes a variety of Code Types to classify these MDRs. One of these code types is Patient Code which is being split into Health Effect – Clinical Codes and Health Effect – Impact Codes.
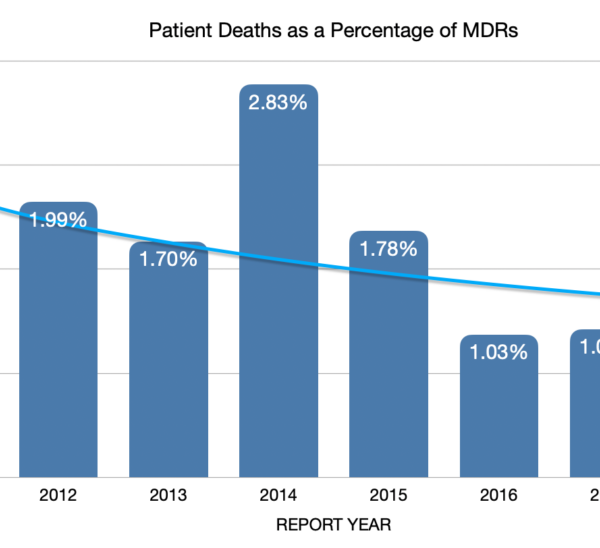
One of the codes used to classify these MDRs is the Patient Death. This code is used to record that the patient died during the course of medical device utilization and it does not mean that the device had anything to do with that death. The data as reported to the FDA is showing that patient deaths are decreasing substantially as a percentage of Medical Device Reports. This could be for several reasons, nevertheless, we think this is significant.
See how Smarteeva can help you make Post Market Surveillance faster and easier by going to our Product Page
About Smarteeva
Based out of Santa Barbara, CA. Smarteeva Software provides cutting edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user friendliness. Built on the Salesforce.com Platform and utilizing the latest innovations in AI and Machine Learning, Smarteeva Software provides a true partner to it’s business users.