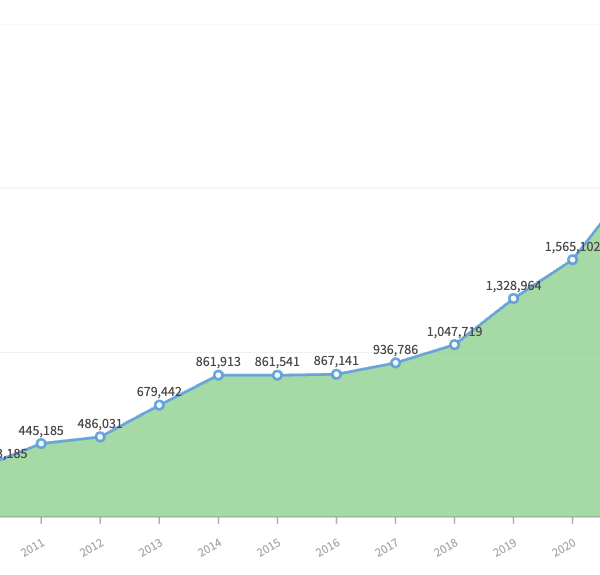
The post-pandemic world has recorded over 3 million MDRs (Adverse Events or serious malfunctions) being reported to the Food and Drug Administration. The long-term trend of 20% annual growth increased by 50% in 2022. When compared to 300,000 in 2010, the recent numbers are staggering.
The Increase in the Volume of Complaints and MDR reports
In the past 10 years, the number of MDRs reported to the FDA has increased at a steady 20% pace annually. In 2010, the number of submitted complaints was 337,838. In 2022, it had increased to over 3 million. Making it a steady rise of almost 50%. The number of complaints filed increased by 20% each year excluding 2016. A conservative estimate shows that 1% of all complaints are MDRs, meaning that there are over 200 million complaints filed annually.
Compliance and Financial Costs of Post-Market Surveillance
A high volume of complaints and MDRs have pronounced implications for businesses. Handling complaints and MDRs is a complex process that goes through multiple stages necessary for their closure, including (Intake, Complaint Evaluations, Investigations, and Root Cause Analysis as well as Regulatory Reporting). The necessity of reviewing MDRs and complaints is painstakingly high, leading to a time crunch for companies to evaluate performance, help with the R&D of new product development, or better communication with regulators.
Managing complaints is not an economical process either. It costs the industry tens of billions of dollars per year. Depending on the device, the money a company may spend on a single MDR can be as high as $2000. These are unsustainable numbers when combined with the cost pressures of both government and private insurance.
Smarteeva’s AI-enabled solutions are state-of-the-art and can help you make Post Market Surveillance faster, and easier. Explore our Product Page.
About Smarteeva
Based out of Santa Barbara, CA. Smarteeva Software provides cutting-edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user-friendliness. Built on the Salesforce platform, utilizing the latest innovations in AI and Machine Learning, Smarteeva Software provides a true partnership to its business users.

