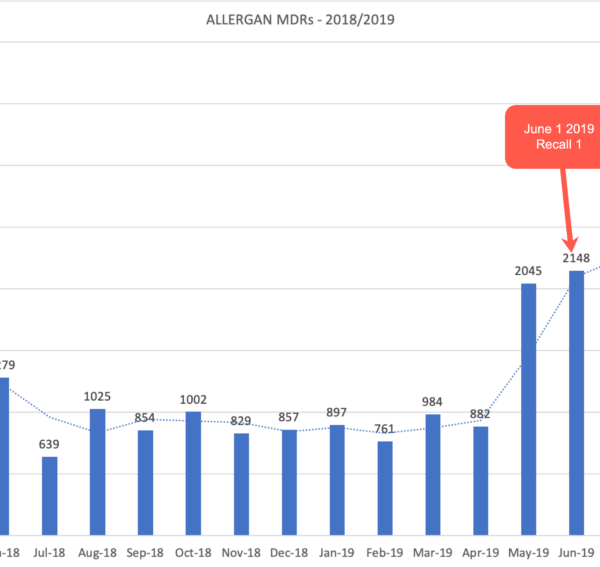
Can MDRs predict when and if a medical device recall will happen? Based on our analysis, one can sometimes tell if a medical device safety recall is about to happen by the sudden big increase in submitted MDRs. As an example, here are 2 separate recalls reported by Allergan in 2019.
Recall 1: Because of greater risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) a Class 1 recall was issued. Class 1 recalls are related to use of products that can result in serious injury or death. The recall letter was issued on June 1, 2019. Prior to that letter, the number of MDRs submitted in May jumped 131% as compared to the month of April. The jump in MDRs can reasonably be associated with the subsequent recall letter.
Recall 2: On November 6, 2019 Allergan issued a Class 3 recall. Class 3 recalls are for reasons that do not involve the risk of serious injury or death. Prior to the recall there was no increase in the number of MDRs submitted to FDA but there was a 92% increase in the month after. No association can be drawn from this recall.
Recall 1 source: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=176152
Recall 2 source: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=178037
Can MDRs predict when and if a medical device recall will happen? Based on our analysis, one can sometimes tell if a medical device safety recall is about to happen by the sudden big increase in submitted MDRs. As an example, here are 2 separate recalls reported by Allergan in 2019.
Recall 1: Because of greater risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) a Class 1 recall was issued. Class 1 recalls are related to use of products that can result in serious injury or death. The recall letter was issued on June 1, 2019. Prior to that letter, the number of MDRs submitted in May jumped 131% as compared to the month of April. The jump in MDRs can reasonably be associated with the subsequent recall letter.
Recall 2: On November 6, 2019 Allergan issued a Class 3 recall. Class 3 recalls are for reasons that do not involve the risk of serious injury or death. Prior to the recall there was no increase in the number of MDRs submitted to FDA but there was a 92% increase in the month after. No association can be drawn from this recall.
Recall 1 source: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=176152
Recall 2 source: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=178037