
The FDA requires Medical Device Reports (MDRs) submission within 30 days of Complaint Awareness and utilizes a variety of code types to classify these MDRs. One of the code types is Patient Code, divided into Health Effect – Clinical Codes & Health Effect – Impact Codes.
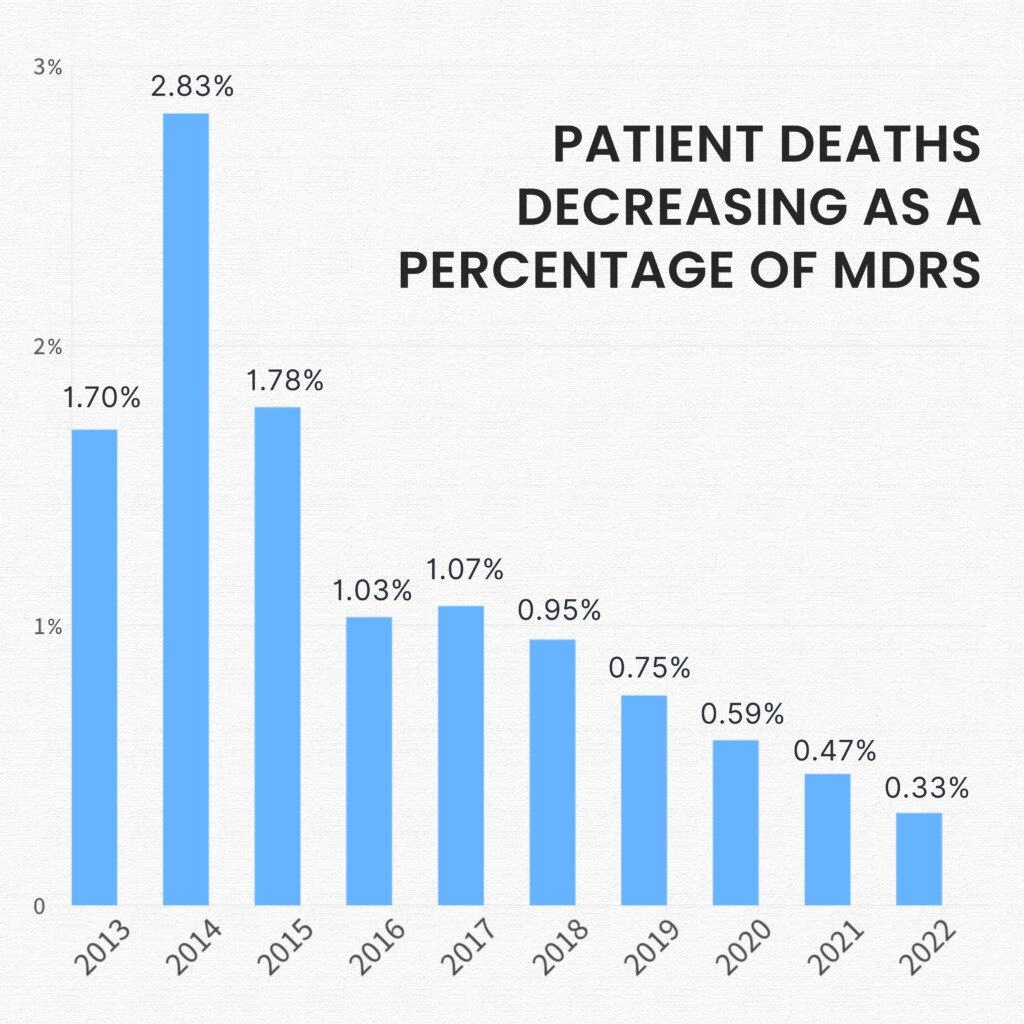
For the past eight years patient death as a percentage of MDRs have been on a constant downward slope. And the trend continued in 2022. While the news is great it’s crucial to know that a patient death mentioned in an MDR does not necessarily link to the usage of the associated medical device.
About Smarteeva:
Based out of Santa Barbara, CA. Smarteeva Software provides cutting-edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user-friendliness. Built on the Salesforce.com Platform and utilizing the latest AI and Machine Learning innovations, Smarteeva Software is a true partner to its business users.
See how Smarteeva can help you make Post Market Surveillance faster and easier by going to our Product Page

