
Smarteeva maintains an internal database of medical device adverse events. We use public data such as MAUDE with other internal and external data sources. This data is cleaned and correlated to make it more user-friendly. We looked at the top MDR submitters in 2022 using our data cleanup technology and found that the top submitters were mostly from consumer-based products such as glucose meters, dental implants and breast implants. It makes sense as the number of devices or procedures performed tends to be orders of higher magnitude.
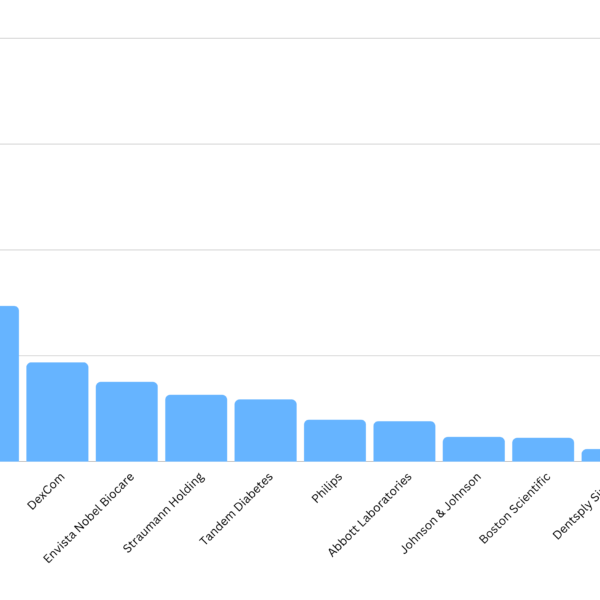
Becton Dickinson was the top MDR submitter in 2022.
Smarteeva’s AI-enabled solutions are state-of-the-art and can help you make Post Market Surveillance faster, and easier. Explore our Product Page.
About Smarteeva:
Based out of Santa Barbara, CA. Smarteeva Software provides cutting-edge and innovative Post Market Surveillance applications for the Medical Device industry. Smarteeva’s Complaint Handling, Adverse Event Reporting, Risk Management and Recall Management solutions prioritize compliance, organizational efficiencies and user-friendliness. Built on the Salesforce platform, utilizing the latest innovations in AI and Machine Learning, Smarteeva Software provides a true partnership to its business users.

